20
Density of matter
WORK AND EXPERIMENTATION TECHNIQUES
To learn basic facts about density and practise using different
laboratory instruments.
Materials
❚
Felt tip pen
❚
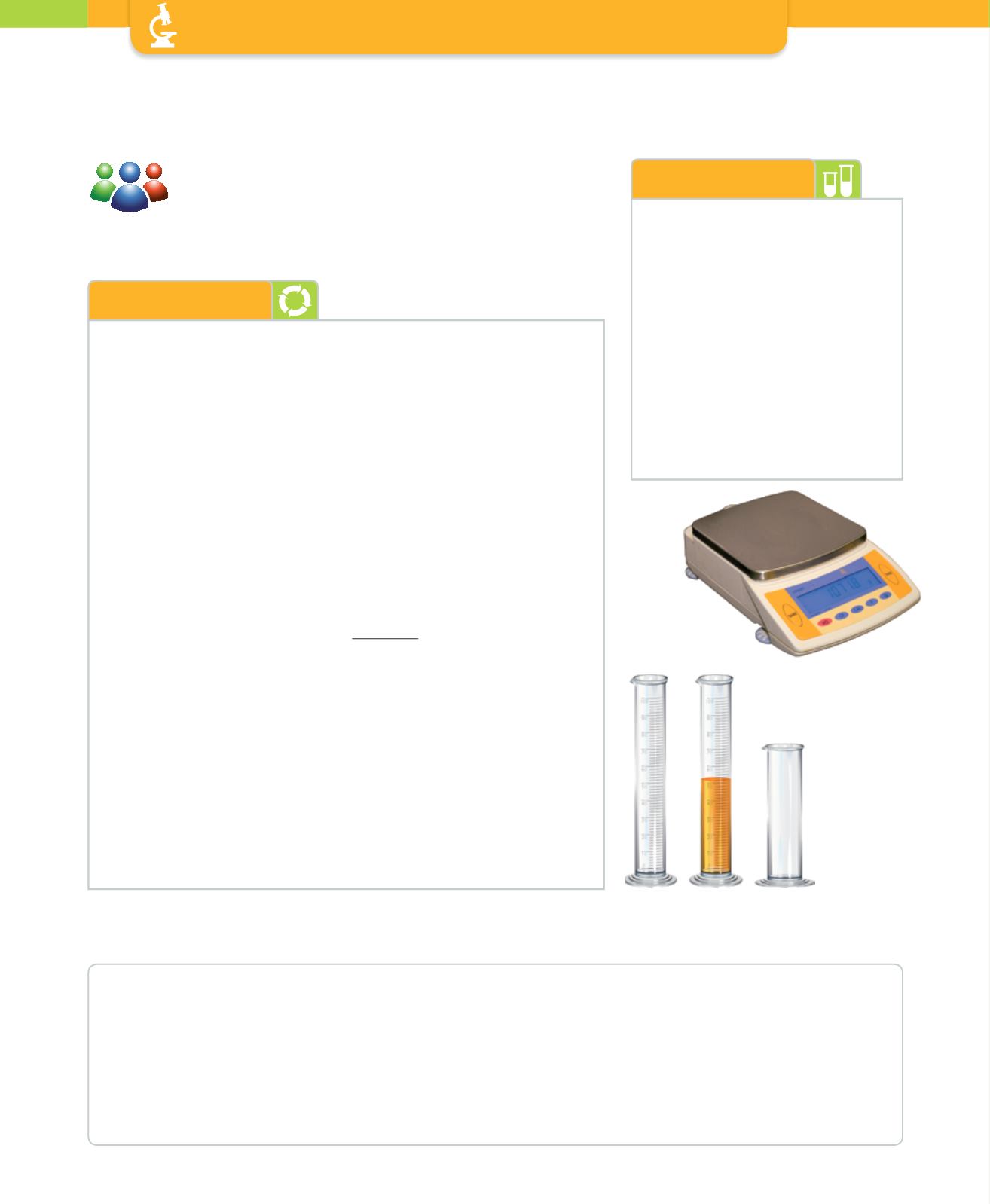
Scales
❚
4 measuring cylinders (100 ml)
❚
1 measuring cylinders (250 ml)
❚
50 ml of water
❚
50 ml of alcohol
❚
50 ml of oil
❚
Cork
❚
A stone (with a smaller mass than
the cork).
1.
Use a felt tip pen to number three of the 100 mL measuring cylinders.
2.
Weigh the measuring cylinders individually and record the mass of each
one in your notebook.
3.
Add 50 mL of water to measuring cylinder 1 and weigh it again. Record
the result and calculate the mass of the 50 mL of water.
4.
Add 50 ml of alcohol to cylinder 2 and weigh it. Record the result and
calculate the mass of the 50 ml of alcohol.
5.
Add 50 ml of oil to cylinder 3 and weigh again. Record the result and
calculate the mass of the 50 mL of oil.
6.
Calculate the density of the water, alcohol and oil using the following
formula:
In order to express density in grams per litre (g/L), convert the millilitres to
litres first.
7.
Slowly add the water, alcohol and oil in that order, to the 250 mL
measuring cylinder. Pour the liquids down the side of the cylinder. Let the
mixture settle and observe what happens.
8.
Weigh the cork and the stone separately and make a note of their masses
in your notebook.
9.
Add 50ml of water to the 100 ml measuring cylinder. Place the cork and
the stone in it and observe what happens.
Procedure
density (g/L) =
volume (L)
mass (g)
1.
Which of the liquids is heavier? Which of them is lighter?
2.
In steps 7 and 8:
a)
Which order did the liquids settle in?
b)
Is there a relationship between the density of the three liquids and the order that they settled in?
c)
If we mix 100 mL of oil, 75 ml of water and 50 ml of alcohol, would the liquids settle in the same order?
3.
Did the cork or the stone float? Why?
4.
Based on the experiments, explain in your own words the difference between mass and density.


